Fabry disease treatment market was valued at USD 2.10 Billion in 2024, registering a CAGR of 10.2% during the forecast period (2024-2032), and is projected to be worth USD 5.03 Billion by 2032. The widespread R&D activities and implementation of cutting-edge technology in research procedures are the main factors propelling the Fabry disease market expansion. The demand for reliable therapy is anticipated to rise as a result of problems in physiological systems like the cardiac, urinary, and other systems, as well as the hereditary nature and severity of the disease. One of the important factors influencing the market is extensive R&D practices.
Fabry Disease Treatment Market Dynamics:
Fabry disease is a rare X-linked lysosomal storage condition that causes progressive organ failure due to an alpha-galactosidase enzyme deficiency. The condition is brought on by an abnormal accumulation of a certain fatty substance known as globotriaosylceramide in the body’s tissues, including the eyes, skin, kidneys, gastrointestinal tract, brain, heart, and central nervous system.
Driver:
A growing patient population with Fabry disease and increased use of cutting-edge treatments like chaperone therapy have fueled the market’s growth. The expansion over the projection period is also expected to be accelerated by intensive R&D activities and probable approval of promising pipeline products, such as substrate reduction therapies and enzyme replacement therapies.
Restraint:
There are several medicinal compounds in the pipeline being studied, including PRX-102, JR-051, NP-003, and GC-1119. However, the market for Fabry illness is expected to be constrained by its uncommon occurrence rate (1 in 40,000), lack of knowledge of genetic science advancements, and technological restrictions in some parts of the world.
COVID-19 Analysis of Fabry Disease Treatment Market:
The Fabry disease treatment market has been severely impacted since the COVID-19 epidemic in many different parts of the world. Shipments were impacted during the initial lockdown due to a labor shortage and the closure of a manufacturing facility. The pandemic’s initial effects on China were tremendous. However, the nation’s circumstances have stabilized, and all product and service production rates have increased. As a result, it is determined that COVID-19’s overall impact on the Fabry disease treatment market is moderate.
Fabry Disease Treatment Market Report Cover:
| Report Attributes | Report Details |
| Study Timeline | 2019-2032 |
| Market Size in 2032 (USD Billion) | 5.03 Billion |
| CAGR (2024-2032) | 10.2% |
| By Treatment | Enzyme Replacement Therapy (ERT), Chaperone Treatment, Substrate Reduction Therapy (SRT), Others |
| By geography | North America– (U.S., Canada, Mexico)
Europe- (Germany, France, U.K., Russia, Italy, Spain, BENELUX, Rest of Europe) Asia Pacific- (China, Japan, India, South Korea, Australia, ASEAN, Rest of Asia Pacific) Latin America- (Brazil, Argentina, Chile, Rest of Latin America) The Middle East and Africa- (GCC, Turkey, Israel, Rest of MEA) |
| Key Players | Shire Plc., Sanofi S.A., Amicus Therapeutics Inc., JCR Pharmaceuticals Co Ltd., ISU Abxis Co Ltd., Idorsia Pharmaceuticals Ltd., Avrobio Inc., Protalix Biotherapeutics Inc., Moderna Therapeutics Inc., and Greenovation Biotech GmbH |
Fabry Disease Treatment Market Segment Analysis:
By Treatment
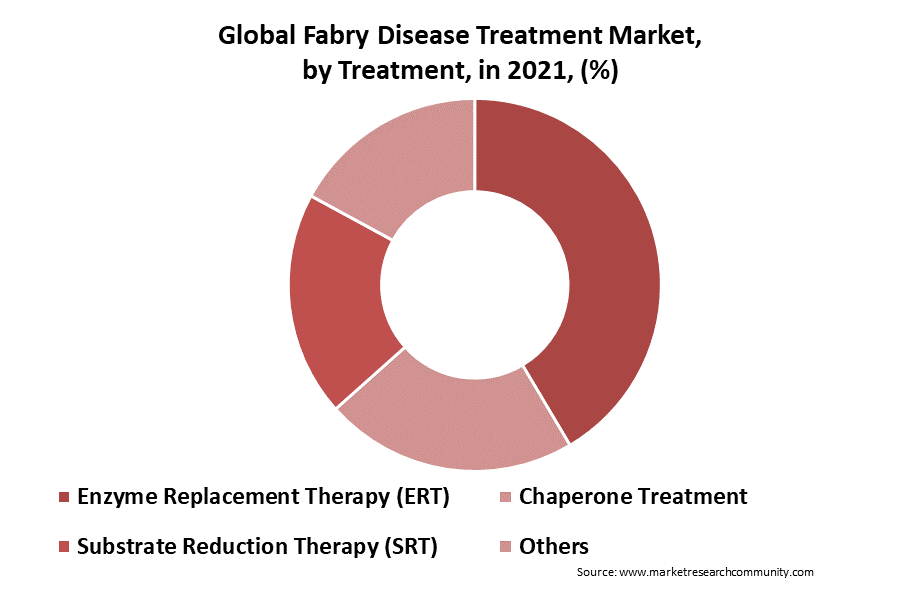
The market for treating Fabry disease based on treatment is segmented into substrate reduction therapy (SRT), chaperone therapy, and enzyme replacement therapy (ERT). Enzyme replacement therapy is the standard of care for the management of illness. Although both ERTs, Fabrazyme from Sanofi and Replagal from the Shire, have received approval in Europe, only Fabrazyme has done so in the United States. Improved safety and efficacy profiles of ERTs and the introduction of novel oral medicines that potentially eliminate the requirement for intravenous infusions are the main goals of current clinical trials. The first oral chaperone medication for adult patients has recently been licensed in the United States, Canada, the European Union, Japan, Australia, Israel, and South Korea for Amicus Therapeutics’ Galafold. Hence, rising regulatory approvals and advancement in medicines are fueling the growth of the Fabry disease treatment market.
By Region
In 2024, North America and then Europe were the two most important regional markets in the Fabry disease treatment market. Improved healthcare facilities, greater adoption of novel therapies, and attractive reimbursement policies are all contributing significantly to the expansion of the industry in the area. Drug companies are increasing R&D expenditures in the area of rare diseases as a result of health insurance schemes covering expensive treatments like Fabrazyme and advantageous government healthcare regulations.
Fabry Disease Treatment Market Competitive Landscape:
The competitive landscape of the market has been analyzed in the report, along with the detailed profiles of the major players operating in the industry. The majority of manufacturers are concentrating on new Treatment launches, improvements to current Treatments, and mergers and acquisitions. The key player in the market include
- Takeda
- Sanofi
- Amicus Therapeutics
- ISU Abaxis
- JCR Pharmaceuticals Co Ltd
- Shire Plc.
- Amicus Therapeutics Inc.
- Idorsia Pharmaceuticals Ltd.
- Avrobio Inc.
- Protalix Biotherapeutics Inc.
- Moderna Therapeutics Inc.
- Greenovation Biotech GmbH
Recent Development
- The Galafold (migalastat) 123 mg capsules were approved by the U.S. Food and Drug Administration (FDA) in August 2018 by Amicus Therapeutics. Adults with Fabry disease can be treated with the oral, precise medication Galafold.
- Protalix Biotherapeutics granted CHIESI Farmaceutici S.p.A. the development and sales rights to PRX-102, an investigational medication for the treatment of Fabry’s disease, in all nations outside of the United States in July 2018.
Table of Content
- Introduction
- Market Introduction
- Market Research Methodology
- Research Process
- Primary Research
- Secondary Research
- Data Collection Technique
- Data Sources
- Market Estimation Methodology
- Limitations of the Study
- Treatment Picture of Fabry Disease Treatment
- Global Fabry Disease Treatment Market: Classification
- Geographic Scope
- Years Considered for the Study
- Research Methodology in brief
- Parent Market Overview
- Overall Fabry Disease Treatment Market Regional Demand
- Research Programs/Design
- Market Breakdown and Data Triangulation Approach
- Data Source
- Secondary Sources
- Primary Sources
- Primary Interviews
- Average Treatment primary breakdown ratio
- Market Dynamics
- Drivers
- Drivers
- Restraints
- Restraints
- Opportunity
- Impact forces on market dynamics
- Impact forces during the forecast years
- Industry Value Chain
- Upstream analysis
- Downstream analysis
- Therapeutic
- Direct Channel
- Indirect Channel
- Potential Customers
- Manufacturing/Operational Cost Analysis
- Pricing Analysis by Region
- Key Treatment Landscape
- Regulatory Analysis
- Porter’s Analysis
- Supplier Power
- Buyer Power
- Substitution Threat
- Threat from New Entry
- Competitive Rivalry
- PESTEL Analysis
- Political Factors
- Economic Factor
- Social Factors
- Technological Factor
- Environmental Factors
- Legal Factor
- Covid-19 impact on Global Economy
- Covid-19 impact on Fabry Disease Treatment Market demand
- Post-Covid Impact on Fabry Disease Treatment Market Demand
- Impact Analysis of Russia-Ukraine Conflict
- Drivers
- Global Fabry Disease Treatment Market Segmentation, Revenue (USD Billion), (2022-2030)
- By Treatment
- Enzyme Replacement Therapy (ERT)
- Chaperone Treatment
- Substrate Reduction Therapy (SRT)
- Others
- By Treatment
- By Global Fabry Disease Treatment Market Overview, By Region
- North America Fabry Disease Treatment Market Revenue (USD Billion), by Countries, (2022-2030)
- US
- By Treatment
- Canada
- Mexico
- US
- Europe Fabry Disease Treatment Market Revenue (USD Billion), by Countries, (2022-2030)
- France
- UK
- Spain
- Russia
- Italy
- BENELUX
- Asia Pacific Fabry Disease Treatment Market Revenue (USD Billion), by Countries, (2022-2030)
- China
- Japan
- Australia
- South Korea
- India
- ASEAN
- North America Fabry Disease Treatment Market Revenue (USD Billion), by Countries, (2022-2030)
- Latin America Fabry Disease Treatment Market Revenue (USD Billion), by Countries, (2022-2030)
- Brazil
- Argentina
- Chile
- The Middle East and Africa Fabry Disease Treatment Market Revenue (USD Billion), by Countries, (2022-2030)
- GCC
- Turkey
- South Africa
- Global Fabry Disease Treatment Market Revenue: Competitive Analysis, 2021
- Key strategies by players
- Revenue (USD Billion and %), By manufacturers, 2021
- Player Positioning by Market Players, 2021
- Competitive Analysis
- Takeda
- Business Overview
- Business Financials (USD Billion)
- Treatment Category, Source, and Specification
- Main Business/Business Overview
- Geographical Analysis
- Recent Development
- SWOT Analysis
- Sanofi
- Amicus Therapeutics
- ISU Abaxis
- JCR Pharmaceuticals Co Ltd
- Shire Plc.
- Amicus Therapeutics Inc.
- Idorsia Pharmaceuticals Ltd.
- Avrobio Inc.
- Protalix Biotherapeutics Inc.
- Moderna Therapeutics Inc.
- Greenovation Biotech GmbH
- Takeda
- Market Research Findings & Conclusion
Disclaimer
Research Methodology
The Market Research Community offers numerous solutions and its full addition in the research methods to be skilled at each step. We use wide-ranging resources to produce the best outcome for our customers. The achievement of a research development is completely reliant on the research methods implemented by the company. We always faithful to our clients to find opportunities by examining the global market and offering economic insights.Market Research Community are proud of our widespread coverage that encompasses the understanding of numerous major industry domains. Company offers consistency in our research report, we also offers on the part of the analysis of forecast across a range of coverage geographies and coverage. The research teams carry out primary and secondary research to carry out and design the data collection methods.
