In Vitro Diagnostics Quality Control Market Insights
In Vitro Diagnostics Quality Control Market is projected to be worth USD 2.27 Billion by 2028, registering a CAGR of 5.1 % CAGR during the forecast period (2022-2028), The market was valued at USD 1.60 Billion in 2021.
In vitro diagnostics quality control is a set of procedures, or a sample/material used to validate to ensure the performance, accuracy, and environmental conditions that affect the IVD test results. The market is growing at a high pace due to the rise in the need for quality controls by third-party, stringent mandates from certified bodies & regulatory agencies concerning the use of controls, and a rise in approved clinical laboratories.
In Vitro Diagnostics Quality Control Market Dynamics
Driver:
A growing sum of accredited clinical laboratories globally and the existence of encouraging regulatory bodies are the key factors fueling the growth of the Vitro diagnostics market.
Restraints:
The additional costs of the QC process and budget restraints in hospitals and laboratories, and stringent product approvals are hindering the growth of the Vitro diagnostics market.
Opportunity:
Technological advancements and the demand for multi-analyte and multi-instrument controls to control consolidate multiple instrument-specific control and single control. This factor is expected to create profitable opportunities for the market.
COVID-19 Analysis of In Vitro Diagnostics Quality Control Market
The In Vitro Diagnostics Quality Control market has been positively impacted since the COVID-19 epidemic in many different parts of the world. With the occurrence of the global pandemic COVID-19 and the requirement for laboratory tests and the higher hospitalization has risen drastically. Further, viral infections have led to more development of these In Vitro Diagnostics Quality Controls as it resulted in quick and early diagnosis of the infections. Therefore, during the pandemic and post-pandemic, the In Vitro Diagnostics Quality Control market has seen profitable growth.
In Vitro Diagnostics Quality Control Market Report Coverage
| Report Attributes | Report Details |
| Study Timeline | 2016-2028 |
| Market Size in 2028 (USD Billion) | 2.27 |
| CAGR (2022-2028) | 5.1% |
| By Type | Quality Control Products, Data Management Solutions, Quality Assurance Services |
| By Application | Immunochemistry, Hematology, Molecular Diagnostics, Coagulation/Hemostasis, Others |
| By End Use | Hospitals, Clinical Laboratories, IVD Manufacturers & CROs, Others |
| By geography | North America: U.S., Canada, Mexico
Europe: Germany, France, U.K., Russia, Italy, Spain, BENELUX, Rest of Europe Asia Pacific: China, Japan, India, South Korea, Australia, ASEAN, Rest of Asia Pacific Latin America: Brazil, Argentina, Chile, Rest of Latin America The Middle East and Africa: GCC, Turkey, Israel, Rest of MEA |
In Vitro Diagnostics Quality Control Market Segmental Analysis
By Type
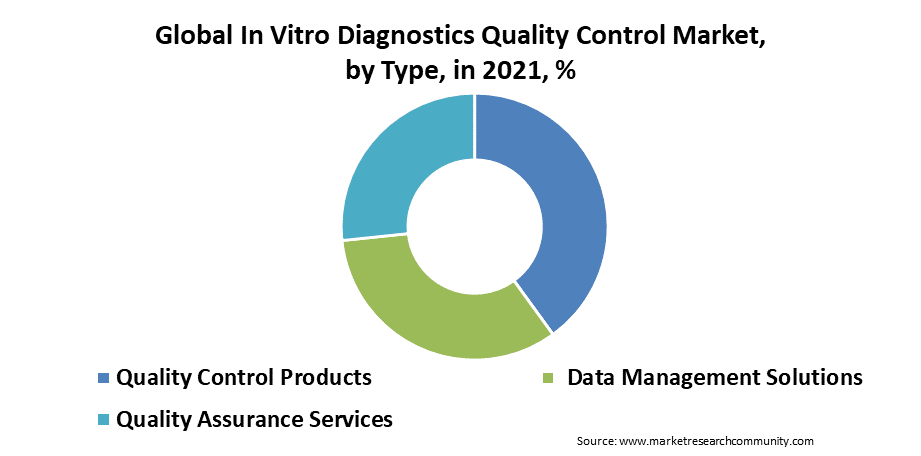
The type segment is categorized into quality control products, data management solutions, and quality assurance services. Quality control products are dominating the type segment in the in vitro diagnostics quality control market. Quality control is the foremost process for monitoring the performance of the clinical laboratory testing process and reagents used in the process to ensure quality precise monitoring is necessary. This in turn drives the growth of the type segment in the in vitro diagnostics quality control market.
By Application
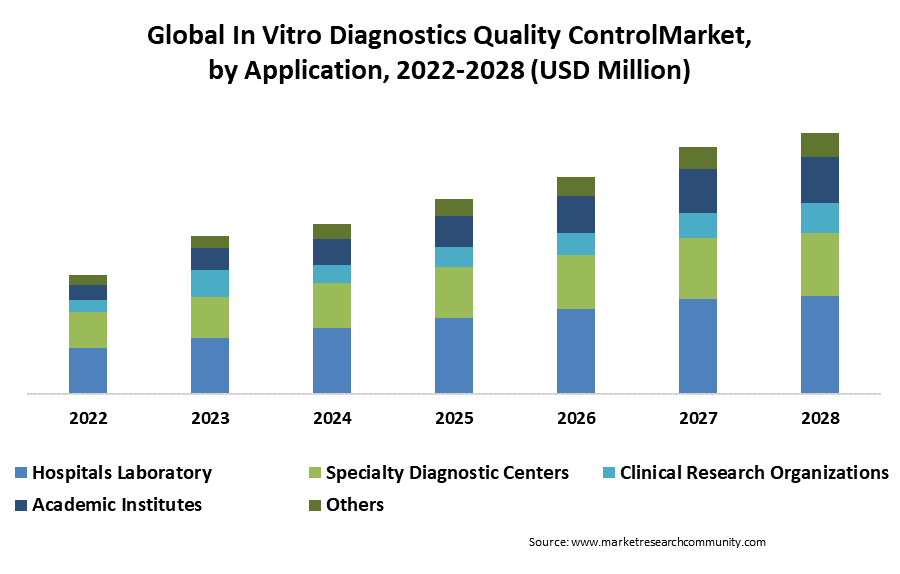
By Application, the segment includes immunochemistry, hematology, molecular diagnostics, coagulation/hemostasis, and others. Immunochemistry application is dominating the growth market expansion during the forecast period. Immunochemistry is used for the detection of various microorganisms such as fungus, viruses, and bacteria. Hence, the need for early diagnosis of the growing prevalence of communicable diseases and chronic diseases is driving the segment expansion in the in vitro diagnosis quality control market.
By End-Use
The segment is divided into Hospitals, Clinical Laboratories, IVD Manufacturers & CROs, and Others. The hospital segment is a potential segment of the in vitro diagnostics quality control market. The factors contributing to the growth of the market are the rising need for fast diagnostic tests and the presence of sophisticated IVD instruments and Reagent kits. Moreover, an increasing collaboration of market players with hospitals to provide regents for QC is anticipated to strengthen the growth of the in vitro diagnostics quality control market.
By Region
The regional segment includes Asia Pacific, Europe, North America, the Middle East, and Africa, Latin America in the in vitro diagnostics quality control market. North America led the growth of the market. Owing to the various recognized diagnostic laboratories with strong QC regulation systems. Further, the presence of the U.S. FDA in the region is driving the growth of the market.
In Vitro Diagnostics Quality Control Market Competitive Landscape
The competitive landscape of the market has been analyzed in the report, along with the detailed profiles of the major players operating in the industry. Further, the surge in Research and Development (R&D), Application innovation, various business strategies, and Type launches have accelerated the growth of the In Vitro Diagnostics Quality Control market.
Key Players
- Roche Diagnostics
- SeraCare Life Science Inc.
- Bio-Rad Laboratories, Inc.
- Siemens Healthcare GmbH
- Alere, Inc.
- Abbott Laboratories, Inc.
- Bio-Techne
- Hologic, Inc. (Gen-Probe)
- Qiagen N.V.
- Quidel Corp.
- Becton, Dickinson, and Company (BD)
- bioMerieux, Inc.
- Sysmex Corp.
- Sero AS
- Thermo Fisher Scientific, Inc.
Table of Content
To check our Table of Contents, please mail us at: [email protected]
Research Methodology
The Market Research Community offers numerous solutions and its full addition in the research methods to be skilled at each step. We use wide-ranging resources to produce the best outcome for our customers. The achievement of a research development is completely reliant on the research methods implemented by the company. We always faithful to our clients to find opportunities by examining the global market and offering economic insights.Market Research Community are proud of our widespread coverage that encompasses the understanding of numerous major industry domains. Company offers consistency in our research report, we also offers on the part of the analysis of forecast across a range of coverage geographies and coverage. The research teams carry out primary and secondary research to carry out and design the data collection methods.
