Preclinical CRO Market Insights:
Preclinical CRO Market is projected to be worth USD 8.82 Billion by 2030, registering a CAGR of 7.5% CAGR during the forecast period (2022-2030), the market was valued at USD 4.60 Billion in 2021. The toxicology testing category accounted for the highest revenue share 24.4% of the worldwide preclinical CRO market in 2021, due to a surge in the outsourcing of noncore preclinical CRO studies and the widespread use of toxicology tests. In 2021, it is anticipated that the segment of biopharmaceutical businesses is expected to hold the highest market share, with 79.8%. North America is expected to hold the greatest share of 47.54% in 2021, due to the presence of reputable CROs with expertise in early drug discovery, such as LabCorp and Charles River Laboratories.
Preclinical CRO Market Dynamics:
Preclinical CROs (contract research organizations) assist new medical product developers to demonstrate their products’ safety and efficacy in live models that the FDA considers approximate as closely as possible the human anatomy before entering clinical trials (or receiving other approvals like 510Ks) or being used for human care.
Driver:
The demand for preclinical contract research organization (CRO) services is increasing as a result of increased R&D expenditure for drug development, which is expected to accelerate the preclinical CRO market expansion over the forecast period. The spike in large molecule preclinical trials and the increasing need to control R&D costs are anticipated to contribute to the rising need for high-quality preclinical CRO.
Opportunities:
The Food and Drug Administration’s procedure for approving drugs has undergone major modification (FDA). The U.S. recently enacted the 21st Century Cures bill, which accelerated the approval procedure for the introduction of ground-breaking pharmaceuticals and medical devices. These modifications to the approval procedures are projected to spur innovation and raise demand for preclinical services, both of which are expected to create profitable opportunities for the preclinical CRO market.
COVID-19 Analysis of Preclinical CRO Market:
The preclinical CRO market has been severely impacted since the COVID-19 epidemic in many different parts of the world. Shipments were impacted during the initial lockdown due to a labor shortage and the closure of a manufacturing facility. The pandemic’s initial effects on China were tremendous. However, the nation’s circumstances have stabilized, and all product and service production rates have increased. As a result, it is determined that COVID-19’s overall impact on the preclinical CRO market is moderate.
Preclinical CRO Market Report Coverage:
| Report Attributes | Report Details |
| Study Timeline | 2016-2030 |
| Market Size in 2030 (USD Billion) | 8.82 Billion |
| CAGR (2022-2030) | 7.5% |
| By Service | Bioanalysis and DMPK studies, Toxicology Testing, Compound Management, Chemistry, Safety Pharmacology, Others |
| By Model Type | Patient-Derived Organoid (PDO) Model, Patient-derived xenograft model |
| By End-Use | Biopharmaceutical Companies, Government and Academic Institutes, Medical Device Companies |
| By geography | North America– (U.S., Canada, Mexico)
Europe- (Germany, France, U.K., Russia, Italy, Spain, BENELUX, Rest of Europe) Asia Pacific- (China, Japan, India, South Korea, Australia, ASEAN, Rest of Asia Pacific) Latin America- (Brazil, Argentina, Chile, Rest of Latin America) The Middle East and Africa- (GCC, Turkey, Israel, Rest of MEA) |
| Key Players | Alexion Pharmaceutical Inc., Grifols SA, Avadel Pharmaceuticals plc. Novartis AG, Pfizer, Inc., AbbVie Inc., F. Hoffmann-La Roche Ltd., GlaxoSmithKline plc, Bausch Health Companies Inc., and Shire plc. Alexion Pharmaceuticals and Astellas Pharma |
Preclinical CRO Market Segment Analysis:
By Service
The toxicology testing category accounted for the greatest revenue share 24.4% of the worldwide preclinical CRO market in 2021, due to a surge in the outsourcing of noncore preclinical CRO studies and the widespread use of toxicology tests. As CROs now have a better ability to conduct toxicology testing, toxicology is one of the important services that are being outsourced to CROs. This segment’s expansion is anticipated to be driven by the increasing rate at which non-core preclinical investigations are being outsourced to CROs and the CROs’ expanding capacity to provide additional value-added services.
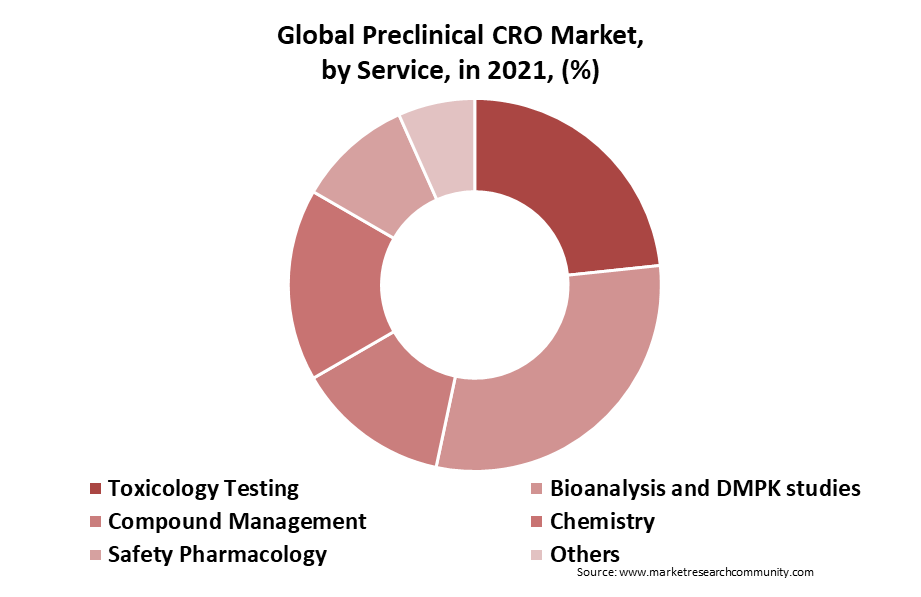
By Model Type
In 2021, the segment with the highest share Patient-Derived Organoid (PDO) Model had a share of 78.34%. The utilization of directly derived cells and tissues from the patient accounts for the patient-derived organoid (PDO) model’s expanding influence. Cryopreserved specimens and improved tailored healthcare are two benefits. They are now a crucial component of preclinical investigations since they facilitate quicker detection and prognosis of cancer.
According to the forecast, the market for patient-derived xenograft models is expected to expand steadily. This is due to more CROs keeping an inventory of immunodeficient mice with patient-derived xenografts on hand (PDXs). Because the tumor cells’ original genetic makeup is preserved, this form of study also enables researchers to correlate laboratory studies with patient findings.
By End Use
In 2021, it is anticipated that the segment of biopharmaceutical businesses is expected to hold the highest market share, with 79.8% in the preclinical CRO market. The need for preclinical CRO services is anticipated to increase in the future due to the mounting inclination of biopharmaceutical businesses to outsource end-to-end services, particularly among small- and mid-size enterprises that lack important proficiency in the preclinical phase of drug development.
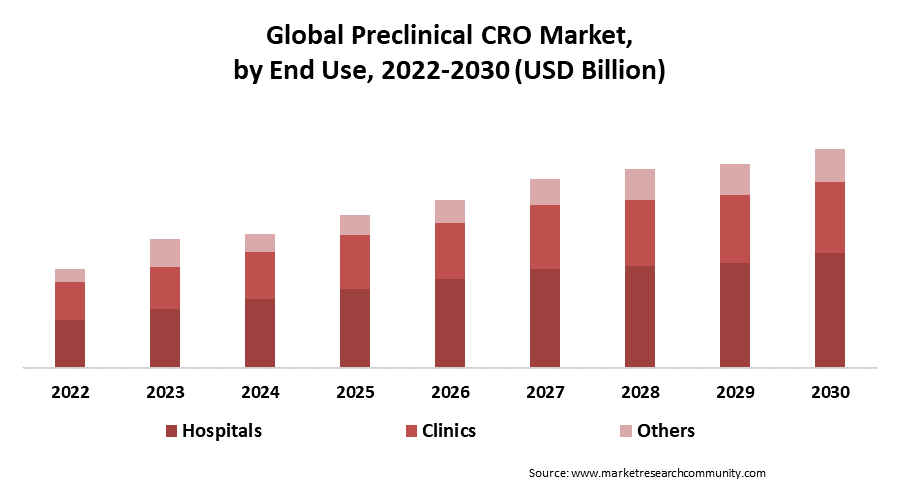
By Region
The regional segment includes Asia Pacific, North America, Europe, the Middle East, Africa, and Latin America. North America is expected to hold the greatest share of 47.54% in 2021, due to the presence of reputable CROs with expertise in early drug discovery, such as LabCorp and Charles River Laboratories. To take advantage of the Investigational New Drug (IND) application, which has been approved by the FDA, numerous biopharmaceutical firms favor outsourcing their preclinical trials to CROs situated in the U.S., making it the largest market for preclinical trial outsourcing.
Preclinical CRO Market Competitive Landscape:
The competitive landscape of the market has been analyzed in the report, along with the detailed profiles of the major players operating in the industry. Further, the surge in Research and Development (R&D), product innovation, various business strategies, and Service & Service launches have accelerated the growth of the preclinical CRO market. Key players in the market include-
- Eurofins Scientific
- PRA Health Sciences, Inc.
- Wuxi AppTec
- Medpace, Inc.
- Charles River Laboratories International, Inc.
- Pharmaceutical Product Development (PPD), LLC
- SGS SA (SGS)
- Intertek Group Plc (IGP)
- Laboratory Corporation of America, Inc.
- Crown Bioscience
Recent Development
- Contract research company Toxikon Corporation was purchased by Labcorp in December 2021. The development and growth of CRO services in pharmaceuticals and medical devices are expected to be aided by this acquisition of Labcorp.
- To give the best antibodies for a variety of disorders, Eurofins Scientific and Fusion Antibodies joined in August 2021. This cooperation has a two-year contract and is expected to make use of cutting-edge technologies.
Table of Content
- Introduction
- Market Introduction
- Market Research Methodology
- Research Process
- Primary Research
- Secondary Research
- Data Collection Technique
- Data Sources
- Market Estimation Methodology
- Limitations of the Study
- Service Picture of Preclinical CRO
- Global Preclinical CRO Market: Classification
- Geographic Scope
- Years Considered for the Study
- Research Methodology in brief
- Parent Market Overview
- Overall Preclinical CRO Market Regional Demand
- Research Programs/Design
- Market Breakdown and Data Triangulation Approach
- Data Source
- Secondary Sources
- Primary Sources
- Primary Interviews
- Average primary breakdown ratio
- Market Dynamics
- Drivers
- Drivers
- Restraints
- Restraints
- Opportunity
- Impact forces on market dynamics
- Impact forces during the forecast years
- Industry Value Chain
- Upstream analysis
- Downstream analysis
- Therapeutic
- Direct Channel
- Indirect Channel
- Potential Customers
- Manufacturing/Operational Cost Analysis
- Pricing Analysis by Region
- Key Service Landscape
- Regulatory Analysis
- Porter’s Analysis
- Supplier Power
- Buyer Power
- Substitution Threat
- Threat from New Entry
- Competitive Rivalry
- PESTEL Analysis
- Political Factors
- Economic Factor
- Social Factors
- Technological Factor
- Environmental Factors
- Legal Factor
- Covid-19 impact on Global Economy
- Covid-19 impact on Preclinical CRO Market demand
- Post-Covid Impact on Preclinical CRO Market Demand
- Impact Analysis of Russia-Ukraine Conflict
- Drivers
- Global Preclinical CRO Market Segmentation, Revenue (USD Billion), (2022-2030)
- By Service
- Bioanalysis and DMPK studies
- In vitro ADME
- In-vivo PK
- Toxicology Testing
- GLP
- Non-GLP
- Compound Management
- Process R&D
- Custom Synthesis
- Others
- Chemistry
- Medicinal Chemistry
- Computation Chemistry
- Safety Pharmacology
- Others
- Bioanalysis and DMPK studies
- By Model Type
- Patient-Derived Organoid (PDO) Model
- Patient-derived xenograft model
- By End-Use
- Biopharmaceutical Companies
- Government and Academic Institutes
- Medical Device Companies
- By Service
- By Global Preclinical CRO Market Overview, By Region
- North America Preclinical CRO Market Revenue (USD Billion), by Countries, (2022-2030)
- US
- By Service
- By Model Type
- By End-Use
- Canada
- Mexico
- US
- Europe Preclinical CRO Market Revenue (USD Billion), by Countries, (2022-2030)
- France
- UK
- Spain
- Russia
- Italy
- BENELUX
- Asia Pacific Preclinical CRO Market Revenue (USD Billion), by Countries, (2022-2030)
- China
- Japan
- Australia
- South Korea
- India
- ASEAN
- North America Preclinical CRO Market Revenue (USD Billion), by Countries, (2022-2030)
- Latin America Preclinical CRO Market Revenue (USD Billion), by Countries, (2022-2030)
- Brazil
- Argentina
- Chile
- Middle East and Africa Preclinical CRO Market Revenue (USD Billion), by Countries, (2022-2030)
- GCC
- Turkey
- South Africa
- Global Preclinical CRO Market Revenue: Competitive Analysis, 2021
- Key strategies by players
- Revenue (USD Billion and %), By manufacturers, 2021
- Player Positioning by Market Players, 2021
- Competitive Analysis
- Alexion Pharmaceutical Inc.
- Business Overview
- Business Financials (USD Billion)
- Service Category, Source, and Specification
- Main Business/Business Overview
- Geographical Analysis
- Recent Development
- Swot Analysis
- Grifols SA
- Avadel Pharmaceuticals plc. Novartis AG
- Pfizer Inc.
- AbbVie Inc.
- Hoffmann-La Roche Ltd.
- GlaxoSmithKline plc
- Bausch Health Companies Inc.
- Shire plc. Alexion Pharmaceuticals
- Astellas Pharma
- Alexion Pharmaceutical Inc.
- Market Research Findings & Conclusion
Disclaimer
Research Methodology
The Market Research Community offers numerous solutions and its full addition in the research methods to be skilled at each step. We use wide-ranging resources to produce the best outcome for our customers. The achievement of a research development is completely reliant on the research methods implemented by the company. We always faithful to our clients to find opportunities by examining the global market and offering economic insights.Market Research Community are proud of our widespread coverage that encompasses the understanding of numerous major industry domains. Company offers consistency in our research report, we also offers on the part of the analysis of forecast across a range of coverage geographies and coverage. The research teams carry out primary and secondary research to carry out and design the data collection methods.
