Biosimilars Market Insights
Biosimilars Market is projected to be worth USD 70.14 Billion by 2028, registering a CAGR of 24.3% CAGR during the forecast period (2024-2028), The market was valued at USD 15.30 Billion in 2024. The monoclonal antibodies segment is anticipated to grab a high share in 2024 due to a surge in R&D activities to develop new biosimilars. The oncology segment is estimated to lead the application segment of the Biosimilars market due to the surge in the prevalence of cancer and the use of biosimilars in the treatment. In the regional segment, Europe is estimated to dominate the growth of the market due emergence of new players and a surge in chronic illness.
A biosimilar is a biological product that closely resembles a reference product. FDA-approved biologics are referred to as the reference product and have been compared to FDA-approved biosimilars. Reference and biosimilar products are typically complex compounds made from living things and are closely inspected to assure high quality.
Biosimilars Market Dynamics
Driver:
Increasing demand for biosimilar drugs due to their cost-effectiveness is projected to drive the Biosimilars market. As biosimilars are priced around 20–30% lower than their parent/branded counterparts.
Restraints:
The development of biosimilars is a highly complex process that requires high investments, technical capabilities, clinical trial expertise, scientific standards, and quality systems. Hence, this factor is estimated to hamper the growth of the Biosimilars market.
Opportunity:
The patent expiry of biological drugs is estimated to bring greater opportunities. As nearly 20 oncology biologics will expire by 2032, which could lead to more biosimilars in cancer care and therefore reduced costs. This factor is estimated to boost opportunities for the key players in the Biosimilars market.
COVID-19 Analysis of Biosimilars Market
The detrimental effects of COVID-19’s global expansion on numerous countries have shocked governments around the world, prompting them to act decisively to stop COVID-19. The first strain of Coronavirus was identified in Wuhan, China in December 2019 which affected the entire world. On a rise in infection across various nations, around 212 countries were infected by the pandemic. Most companies in numerous nations have been severely impacted by the global crisis COVID-19 pandemic. Import-export limitations that caused delays and disturbances at foreign borders harmed the Biosimilars market as well. The Biosimilars market supply chain faced numerous obstacles as a result of restrictions on the mobility of individuals and goods. Hence, overall, the Biosimilars market has observed a moderate impact on the economy.
Biosimilars Market Report Coverage
| Report Attributes | Report Details |
| Study Timeline | 2016-2028 |
| Market Size in 2028 (USD Billion) | 70.14 |
| CAGR (2024-2028) | 24.3% |
| By Type | Human growth hormone, Erythropoietin, Monoclonal antibodies, Insulin, Granulocyte-Colony Stimulating Factor, Others |
| By Application | Blood disorders, Oncology diseases, Chronic and autoimmune diseases, Others |
| By geography | North America: U.S., Canada, Mexico, Europe, Germany, France, U.K., Russia, Italy, Spain, BENELUX, Rest of Europe,
Asia Pacific: China, Japan, India, South Korea, Australia, ASEAN, Rest of Asia Pacific Latin America: Brazil, Argentina, Chile, Rest of Latin America The Middle East and Africa: GCC, Turkey, Israel, Rest of MEA |
Biosimilars Market Segment Analysis
By Type
The type segment is categorized into human growth hormone, erythropoietin, monoclonal antibodies, insulin, granulocyte-colony stimulating factor, and others. The monoclonal antibodies segment is anticipated to dominate the biosimilars market share in 2024 and is anticipated to grow throughout the forecast period. The factors responsible for the growth of the market are an increase in R&D activities to develop new biosimilars and a rise in the use of monoclonal antibodies for cancer treatment. Hence, the above-mentioned factors are estimated to surge the demand for monoclonal antibodies in the Biosimilars market.
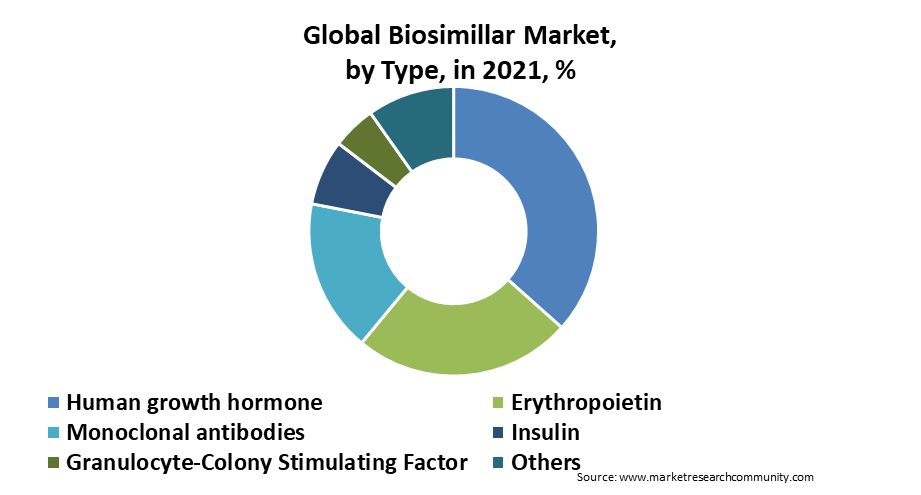
By Application
The oncology segment accounted for the largest market share in 2024 due to the increasing prevalence of cancer. For instance, according to the International Agency for Research on Cancer (IARC), approximately 14.1 million new cancer cases were reported worldwide in 2012, which is expected to rise to approximately 21.7 million by 2032. Cancer-related deaths are higher in undeveloped and developing countries than in wealthy nations. Further, the availability of biosimilar drugs at low cost than other branded drugs is estimated to boost the oncology segment in the Biosimilars market expansion.
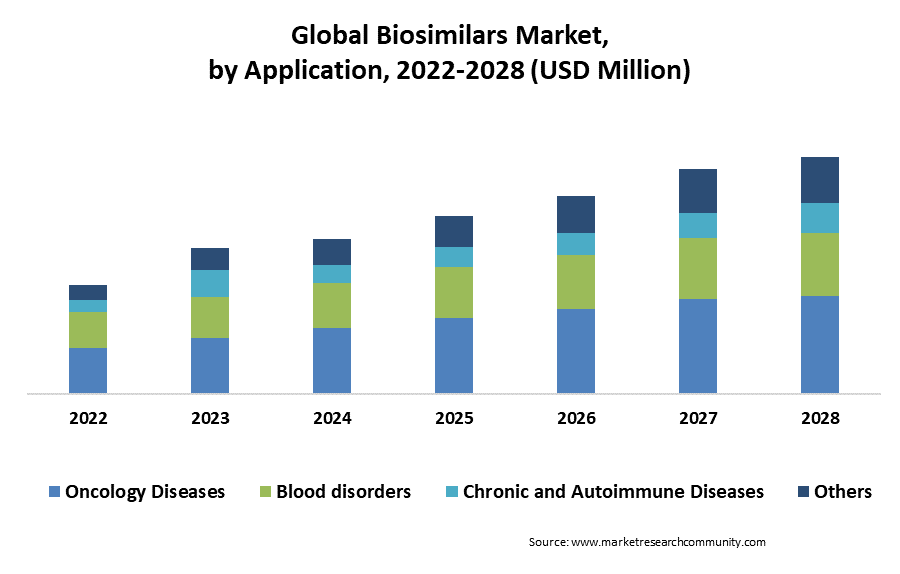
By Region
The regional segment includes Asia Pacific, Europe, North America, Middle East, and Africa, Latin America. Europe held the greatest market share for biosimilars in 2024, followed by North America, Asia Pacific, and South America. The impending patent expiration of biologic drugs and the introduction of new biosimilars are expected to boost the biosimilar market in Europe. Further, the rise in the prevalence of chronic diseases, the emergence of new companies, and surging R&D are some of the main drivers for growth in regional markets.
Biosimilars Market Competitive Landscape
The competitive landscape of the market has been analyzed in the report, along with the detailed profiles of the major players operating in the industry. Further, the surge in Research and Development (R&D), Application innovation, various business strategies, and product launches have accelerated the growth of the market.
Key Players
- Pfizer, Inc.
- Reddy’s Laboratories Ltd.
- Amgen, Inc.
- Eli Lilly and Company
- Teva Pharmaceutical Industries Ltd
- Fresenius SE & Co. KGaA
- STADA Arzneimittel AG
- Boehringer Ingelheim
- Gedeon Richter PLC
- Celltrion
- Samsung Biologics
- Coherus BioSciences
- Biocon Limited
- Viatris, Inc.
- Amega Biotech
- Apotex, Inc.
- Biocad
- mAbxience
- Probiomed S.A. De C.V.
- Fujifilm Kyowa Kirin Biologics Co., Ltd.
- Intas Pharmaceuticals Ltd.
- Thermex
- Reliance Life Sciences
- Kashiv Biosciences
Recent Development
- In 2024, the FDA approved SEMGLEE (insulin glargine-yfgn injection), the first interchangeable biosimilar drug for the treatment of diabetes developed by Biocon Ltd (Biocon Biologics) and Viatris, Inc.
- In 2024, Bio-Thera Solutions and Novartis AG companies signed a contract for the commercialization of BAT1706 (Bio-Thera Solutions’ proposed bevacizumab biosimilar) in the US, Europe, Canada, and other international markets.
Table of Content
To check our Table of Contents, please mail us at: [email protected]
Research Methodology
The Market Research Community offers numerous solutions and its full addition in the research methods to be skilled at each step. We use wide-ranging resources to produce the best outcome for our customers. The achievement of a research development is completely reliant on the research methods implemented by the company. We always faithful to our clients to find opportunities by examining the global market and offering economic insights.Market Research Community are proud of our widespread coverage that encompasses the understanding of numerous major industry domains. Company offers consistency in our research report, we also offers on the part of the analysis of forecast across a range of coverage geographies and coverage. The research teams carry out primary and secondary research to carry out and design the data collection methods.
